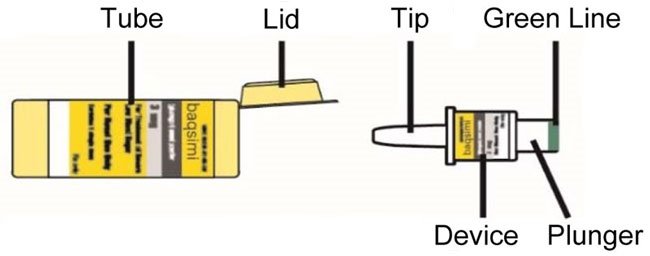
Indication and Description
BAQSIMI™ is indicated for the treatment of severe hypoglycemia in patients with diabetes ages 4 years and above. BAQSIMI contains glucagon, an antihypoglycemic agent used to treat severe hypoglycemia.
Dosage in Adults and Pediatric Patients Aged 4 Years and Above
The recommended dose of BAQSIMI is 3 mg administered as one actuation of the intranasal device into one nostril. If there has been no response after 15 minutes, an additional 3 mg dose of BAQSIMI from a new device may be administered while waiting for emergency assistance.
BAQSIMI is for intranasal use only.
Emphasize the following instructions to the patient or caregiver:
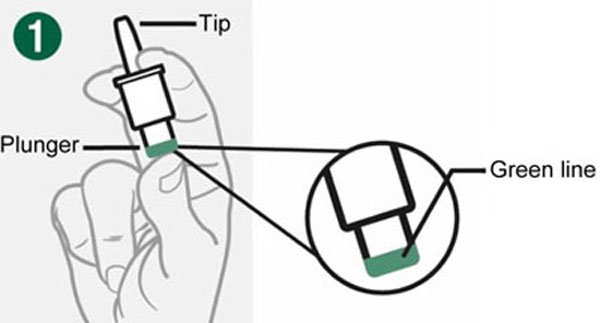
- Do not push the plunger or test the device prior to administration.
- Administer BAQSIMI according to the printed instructions on the shrink-wrapped tube label and the Instructions for Use.
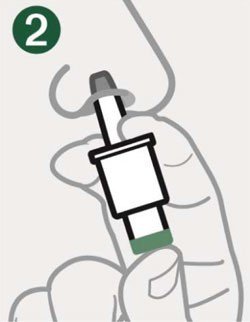
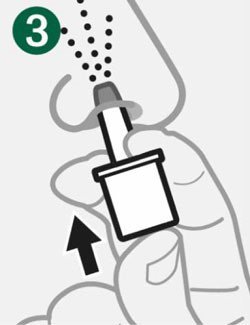
- Administer the dose by inserting the tip into one nostril and pressing the device plunger all the way in until the green line is no longer showing. The dose does not need to be inhaled.
- When the patient responds to treatment, give oral carbohydrates to restore the liver glycogen and prevent recurrence of hypoglycemia.
- Do not attempt to reuse BAQSIMI. Each BAQSIMI device contains one dose of glucagon and cannot be reused.
Important Information to Know
- Do not remove the Shrink Wrap or open the Tube until you are ready to use it.
- If the Tube has been opened, BAQSIMI could be exposed to moisture. This could cause BAQSIMI not to work as expected.
- Do not push the plunger or test BAQSIMI before you are ready to use it.
- BAQSIMI contains 1 dose of glucagon nasal powder and cannot be reused.
- BAQSIMI is for nasal (nose) use only.
- BAQSIMI will work even if you have a cold or are taking cold medicine.
- Baqsimi is passively absorbed in the nasal mucosa, no inhalation required.
Contraindication in patients with:
- Pheochromocytoma [see Warnings and Precautions (5.1)]
- Insulinoma [see Warnings and Precautions (5.2)]
- Known hypersensitivity to glucagon or to any of the excipients in BAQSIMI. Allergic reactions have been reported with glucagon and include anaphylactic shock with breathing difficulties and hypotension [see Warnings and Precautions (5.3)]
Adverse Reactions
Nausea, Headache, Vomiting, Upper respiratory tract irritation.
Drug Interactions
- Beta-blockers: Patients may have a transient increase in pulse and blood pressure when given BAQSIMI.
- Indomethacin: may have a transient increase in pulse and blood pressure when given BAQSIMI.
- Warfarin: BAQSIMI may increase the anticoagulant effect of warfarin.
Overdosage
If overdosage occurs, the patient may experience nausea, vomiting, inhibition of GI tract motility, increase in blood pressure and pulse rate. In case of suspected overdosing, serum potassium levels may decrease and should be monitored and corrected if needed. If the patient develops a dramatic increase in blood pressure, phentolamine mesylate has been shown to be effective in lowering blood pressure for the short time that control would be needed.
Storage and Handling
- Do not remove the Shrink Wrap or open the Tube until you are ready to use it.
- Store BAQSIMI in the shrink-wrapped Tube at temperatures up to 86º F (30ºC ).
- Replace BAQSIMI before the expiration date printed on the Tube or carton.
Be sure to check with your local HealthDirect pharmacy regarding Med D coverage.
For more information visit www.baqsimi.com
References:




