An estimated 2 million older adults in the US have been taking benzodiazepines for more than 120 days1. Benzodiazepines are used for many conditions including anxiety, insomnia, seizures, muscle spasms, and panic disorders. Nonetheless, they are associated with several safety concerns. The 2023 AGS Beers Criteria for potentially inappropriate medication use in older adults states, “Older adults have increased sensitivity to benzodiazepines and decreased metabolism of long-acting agents; the continued use of benzodiazepines may lead to clinically significant physical dependence2. In general, all benzodiazepines increase the risk of cognitive impairment, delirium, falls, fractures, and motor vehicle crashes in older adults.” They further add, “Concomitant use of opioids may result in profound sedation, respiratory depression, coma, and death.” It is important to periodically assess continued need and risk vs benefits of continuing therapy.
The following are practice pearls from the Joint Clinical Practice Guideline (2025)3 that was developed by various medical and professional societies. It applies to patients that have been taking benzodiazepines regularly and may be at risk for physical dependence. It does not apply to patients that are palliative and/or end-of-life care. For more information on patients with substance use disorders (SUD), please see the full guideline as this information is not included in this document.
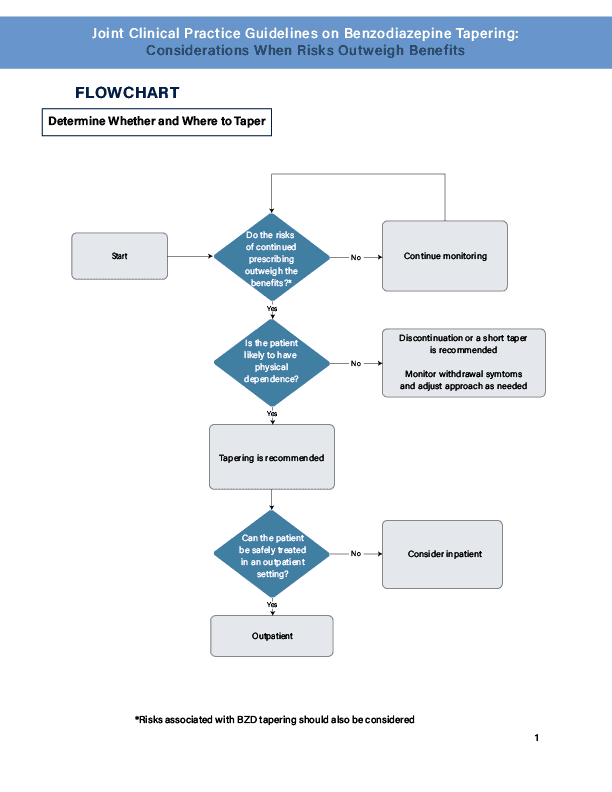
- Risks vs benefits of ongoing Benzodiazepine (BZD) use should be assessed at least every 3 months for each patient taking a BZD. Tapering is generally indicated when the risks outweigh the benefits of continuing.
- Clinicians should generally taper BZD medication in older adults unless there are compelling reasons for continuation (Clinical Consensus, Strong Recommendation). Smaller dose reductions and slower tapers may be required in this population due to metabolic changes, making them more sensitive.
- The Golden Rule: Start LOW and go SLOW!
- Abrupt discontinuations should be avoided in those who are likely to be physically dependent, as abruptly stopping can cause serious and potentially life-threatening withdrawal symptoms.
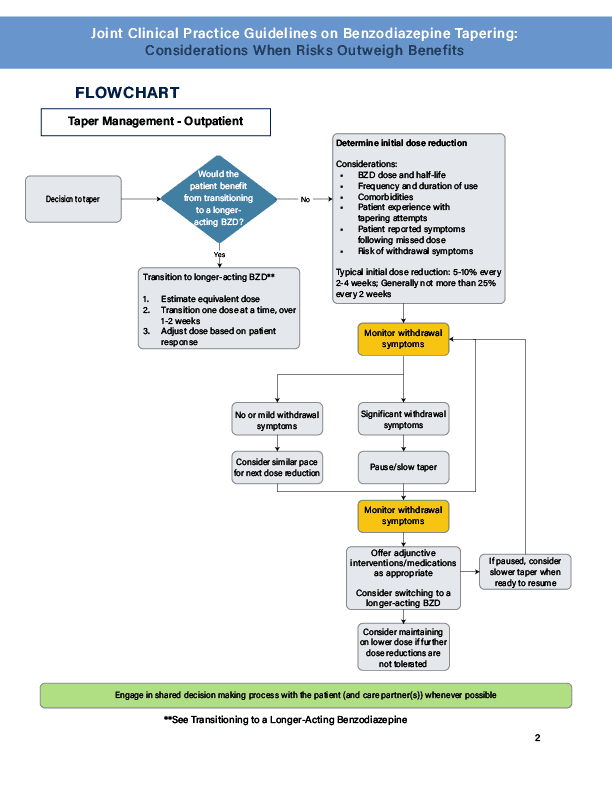
- Consider an initial 5-10% dose reduction every 2-4 weeks. The pace of the taper should not exceed 25% every 2 weeks.
- For those who are likely to have a strong physical dependence (eg, those who have been taking a high dose for more than a year), a slower taper should be considered.
- For those who are unlikely to have significant physical dependence (ie, patients who have been taking a lower dose for a shorter period of time (eg less than 3 months, a taper at the higher end of the dose reduction range (ie, 10-25%) can be considered.
- The goal of tapering may be to completely discontinue the BZD medication, or reduce the dose to the point where the risks no longer outweigh the benefits.
- Each patients taper should be individualized, taking into account how long they have been taking the medication, the dose, how often they are taking the medication, and the medications pharmacokinetics. Please see table below for more information on the pharmacokinetics of common benzodiazepines seen in the LTC setting.
- Cognitive behavioral therapy should be considered to support a successful taper.
- Be aware of common benzodiazepine withdrawal signs and symptoms and how to manage them
- Signs and Symptoms:
- General: headaches, sweating
- Affective: anxiety, panic attacks, depression, agitation, aggression
- Cardiovascular: HTN, chest pain, tachycardia, palpitations
- GI: abdominal cramps, diarrhea, nausea, vomiting
- Neuro: confusion, delirium, cognitive impairment, seizures, sensory hypersensitivity, tingling, tinnitus
- Neuromuscular: balance problems, kinetic disorders, muscle pain and/or jerks, twitches, tremors
- Neuropsychiatric: akathisia, restlessness, psychosis, suicidality
- Sleep: hypersomnia, insomnia, nightmares
- Ways to manage:
- For mild-moderate withdrawal symptoms
- Adjust the tapering schedule (pause or slow the pace).
- Incorporate adjunctive psychosocial interventions.
- No single medication had enough data to support recommending it for withdrawal symptoms. The primary clinical strategy for supporting an effective taper is to first pause or slow the taper in an effort to minimize polypharmacy. If this fails, consideration can be made to use adjunctive medications to manage the symptoms and should be made on a case by case basis.
- Avoid rapid reversal agents such as flumazenil as they carry an increased risk of refractory seizure, cardiac dysrhythmias, and other adverse effects.
- For management of severe or complicated withdrawal symptoms, see full guideline.
- For mild-moderate withdrawal symptoms
- Signs and Symptoms:
| Anxiolytic | Usual Dose in Elderly-Anxiety | Onset of Action (min) | Time to Peak Plasma Level (h) | Half Life (T1/2) in Elderly | FDA Approved Indications |
|---|---|---|---|---|---|
| LONG ACTING | |||||
| Chlordiazepoxide (Librium) | 5mg bid-qid | 15-30 | 0.5-4 hours | 5-30 hours 14-100 hours for active metabolite **should avoid if possible d/t long-acting metabolite | Anxiety Alcohol withdrawal |
| Clonazepam (Klonopin) | Initial: 0.25mg bid Maintenance: 1mg daily Initiate low doses and observe closely | 15-30 | 1-2 h | 30-40 hours | Panic disorder Seizure disorder |
| Diazepam (Valium) | Initial: 2-2.5mg daily-bid | ≤15 | 0.5-2 h | 20-60 hours T1/2 of active metabolite 30-100 hours | Anxiety Acute alcohol withdrawal Seizures Muscle spasms |
| SHORT-INTERMEDIATE ACTING | |||||
| Alprazolam (Xanax) | Immediate release (IR): 0.25 mg bid-tid | 15-30 | 1-2 h | 11.2 hours (range: 6.3-26.9 hours) | Anxiety GAD Panic disorder |
| Lorazepam (Ativan) | Initial: 1-2 mg daily in 2-3 divided doses | 15-30 | 2-4 h | ~14 hours | Anxiety Seizures Insomnia d/t anxiety |
*All of the above information is for oral administration
EMPOWER Brochure – Sleeping Pills & Anti-anxiety Medications (12 pages)
References:
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142. doi:10.1001/jamapsychiatry.2014.1763
- 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;1‐30. doi:10.1111/jgs.18372
- Clinical Guideline Committee (CGC) Members, & ASAM Staff and Contractors. (2025). Joint Clinical Practice Guideline on Benzodiazepine Tapering: Considerations When Risks Outweigh Benefits. Journal of General Internal Medicine, 40(12), 2814–2859. https://doi.org/10.1007/s11606-025-09499-2
- Clinical Pharmacology. Accessed January 2026