Two mRNA vaccines have been granted Emergency Use Authorization (EUA) for use in the United States to protect against COVID-19. mRNA vaccines or messenger RNA (ribonucleic acid) vaccines are a new type of vaccine. Although they are newly approved vaccines, researchers have been studying and working with them for decades. Recent technological advancements have made their use possible. They have been tested in animals against viral diseases including influenza, Zika, rabies, and others. They are even being studied for cancer!1
How mRNA vaccines work
Many of the vaccines that are currently approved to protect us against infectious diseases work by putting a weakened or inactivated germ or “pathogen” into our bodies. This in turn triggers an immune response that includes producing antibodies that protect us from getting infected if we encounter the real virus. mRNA vaccines work a little differently than traditional vaccines. mRNA vaccines do not use any part of the virus that causes COVID-19. Below is a diagram of the SARS-CoV-2 (2019-nCoV) proteins.
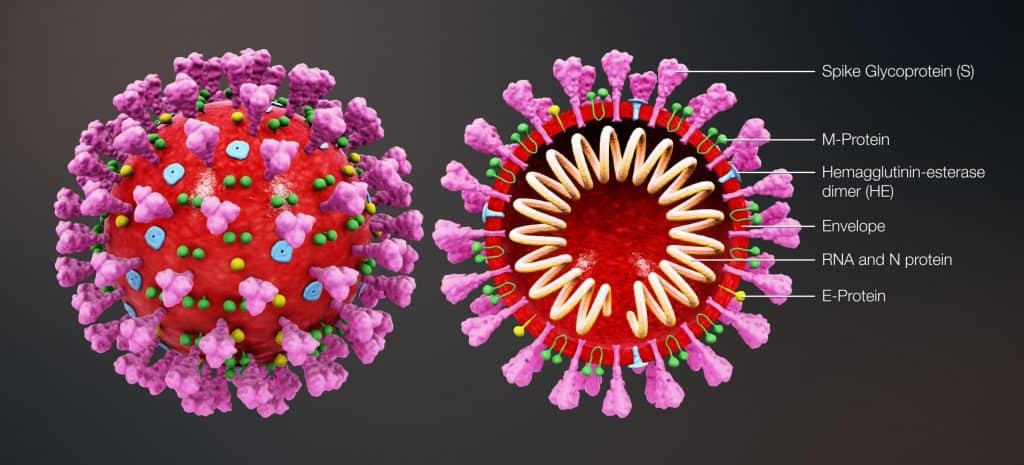
The “spike protein” that is found on the surface of the virus that causes COVID-19. For the virus to gain entry to the inside of a cell, it uses these spike proteins to fuse its own membrane to that of the cells’ and take over the cell. The COVID-19 mRNA vaccines give instructions for our cells to make a harmless piece of this spike protein. mRNA vaccines do not affect or interact with our DNA in any way because they never enter the nucleus of the cell, which is where our DNA is kept. The cell breaks down and gets rid of the mRNA soon after it is finished using the instructions. After our cells have made the spike protein, they display them on their surface. Our immune system recognizes that the protein is foreign and does not belong and will begin building an immune response which includes making antibodies. Through this process, our bodies will have learned to protect us against future infection.2 It is important to note that this process can take a few weeks after vaccination, so it is possible that a person could be infected with the virus that causes COVID-19 just before or just after vaccination and then get sick because the vaccine did not have enough time to provide protection. The vaccine cannot give you COVID-19.
Safety of mRNA vaccines
The vaccines had to go through the same rigorous safety and effectiveness standards as other types of vaccines. Each phase of development was conducted: animal testing, an initial human phase, a second for safety testing, and a third larger phase for efficacy were all completed. Usually, these phases are completed sequentially, meaning one phase is completed before moving onto the next. During the development of the mRNA vaccines, some of these phases were completed in parallel, or at the same time. This helped speed up the development process a bit.3
Speed to Market
Traditional vaccines take quite a bit of time to develop. They involve growing large amounts of the virus, and then weakening the virus or extracting the critical piece. mRNA vaccines, however, are relatively easy to make in the laboratory, and in large amounts.
There were a couple of other factors that contributed to the ability to develop these vaccines quicker than previous vaccines. Usually, production does not start until the studies have shown that the vaccine is both safe and effective. With the mRNA vaccines, companies started producing them before there was evidence they would work. The other factor that accelerated development was the large number of cases circulating. The increased exposures helped accelerate the results of the efficacy trials.
Our Commitment to Protection From COVID-19
HealthDirect is honored to have become one of eight CDC approved providers of the ground-breaking COVID-19 vaccines. As the COVID-19 vaccination program continues to unfold, the wellness resident residing in Senior Care and Special Needs Communities remain our top priority. No matter the new challenges and opportunities, our team of dedicated employee-owners will continue to relentlessly seek and find ways to support our client and their residents in the fight against COVID-19. Check out our COVID-19 page to learn about our participation in Operation Warp Speed and our process for deployment of vaccination clinics.
References:
- Pardi, N., Hogan, M., Porter, F. et al. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov 17, 261–279 (2018).
- Understanding mRNA COVID-19 Vaccines | CDC
- COVID-19 Vaccines: Preparing for Patient Questions – Medscape – Dec 11, 2020.




