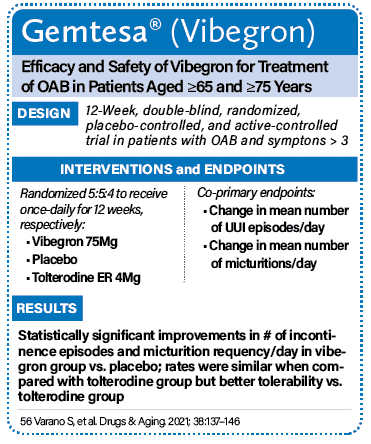
Description:
Vibegron is a selective beta-3 adrenergic agonist. Like mirabegron, vibegron relaxes detrusor smooth muscle and increases bladder capacity, reducing incontinence.
Indication & Dosage:
For the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urinary
urgency, and urinary frequency.
Oral dosage:
- Adults: 75 mg PO once daily
- Adults & Geriatric Maximum Daily Dose: 75 mg/ day PO
Administration:
Administer with or without food. Swallow tablets whole with a glass of water. 75mg Tablets may be crushed
and mixed with a tablespoonful (~15 mL) of applesauce. Swallow applesauce mixture immediately after
preparation, followed by a glass of water.
No titration required.
Warnings/ Precautions/ Disease-related concerns:
- Bladder flow obstruction:
Use with caution in patients with bladder outlet obstruction and in patients using concomitant muscarinic antagonists; may increase the risk of urinary retention. Monitor for signs and symptoms of urinary retention; discontinue use if urinary retention occurs.
Adverse Reactions:
Endocrine & metabolic: Hot flash (<2%),
Gastrointestinal: Constipation (<2%), diarrhea (2%), nausea (2%), xerostomia (<2%)
Genitourinary: Increased post-void residual urine volume (<2%), urinary retention (<2%),
Nervous system: Headache (4%),
Respiratory: Nasopharyngitis (3%), upper respiratory tract infection (2%)
Postmarketing: Dermatologic: Eczema, pruritus, skin rash
Special Populations:
- Patients with Hepatic Impairment Dosing:
Mild to moderate hepatic impairment (Child-Pugh A and B): No dosage adjustment is needed.
Severe hepatic impairment (Child-Pugh C): Use is not recommended; vibegron has not been
studied in this population.
- Patients with Renal Impairment Dosing:
eGFR 15 mL/minute/1.73 m2 or more: No dosage adjustment is needed. eGFR 0 to 14 mL/minute/1.73
m2 (with or without hemodialysis): Use not recommended; vibegron has not been studied in this population.
Dosage Form/Storage:
Thirty (30) 75mg tablets in a 60 cc HDPE bottle with a child-resistant cap, NDC 73336-075-30 Ninety (90) tablets in a 60 cc HDPE bottle with a child-resistant cap, NDC 73336-075-90
Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F)
FOR COMPLETE PRESCRIBING INFORMATION:
medinfo@urovant.com – CALL: 1-833-UROVANT
Reference:
66190 – Gemtesa (vibegron) tablets package insert. Irvine, CA: Urovant Sciences